December 1, 2025
CDI Tip: ICANS Grading and Accurate Documentation
Understanding ICANS in CAR T-Cell Therapy Neurotoxicity
Immune effector cell–associated neurotoxicity (ICANS) is a common and potentially severe complication of CAR T cell therapy neurotoxicity and other immune effector cell therapies. It occurs in 20–60% of CAR T-cell recipients and may range from mild confusion to life-threatening cerebral edema.
For CDI specialists, accurate ICANS grading using standardized criteria is essential for correct ICD-10-CM assignment, severity capture, risk adjustment, and quality reporting.
Pathophysiology and Risk Factors
ICANS is believed to result from cytokine-mediated inflammation, immune activation, and changes in blood–brain barrier permeability. Documentation should reflect contributing factors, including:
- Cytokine Release Syndrome (CRS)
- Preexisting neurological dysfunction
- Tumor burden
- Elevated LDH or ferritin
- High CAR T-cell dose
- Younger patient age
- Intensive lymphodepleting therapy
These factors increase susceptibility to neurotoxicity associated with CD19 targeted CAR T cell therapies.
Clinical Indicators for CDI Review
Ensure that provider documentation includes relevant indicators of neurotoxicity:
- Confusion, somnolence, slowed cognition
- Seizures (clinical or EEG-confirmed)
- Cranial nerve palsies, motor weakness
- Elevated intracranial pressure (ICP)
- Cerebral edema or posturing
These findings support accurate ICANS grading and ICD-10 coding.
The ICANS Grading System (ICE Score)
ICANS is evaluated using the ICE score, which assesses orientation, naming, commands, handwriting ability, consciousness level, motor deficits, seizures, and signs of increased ICP.
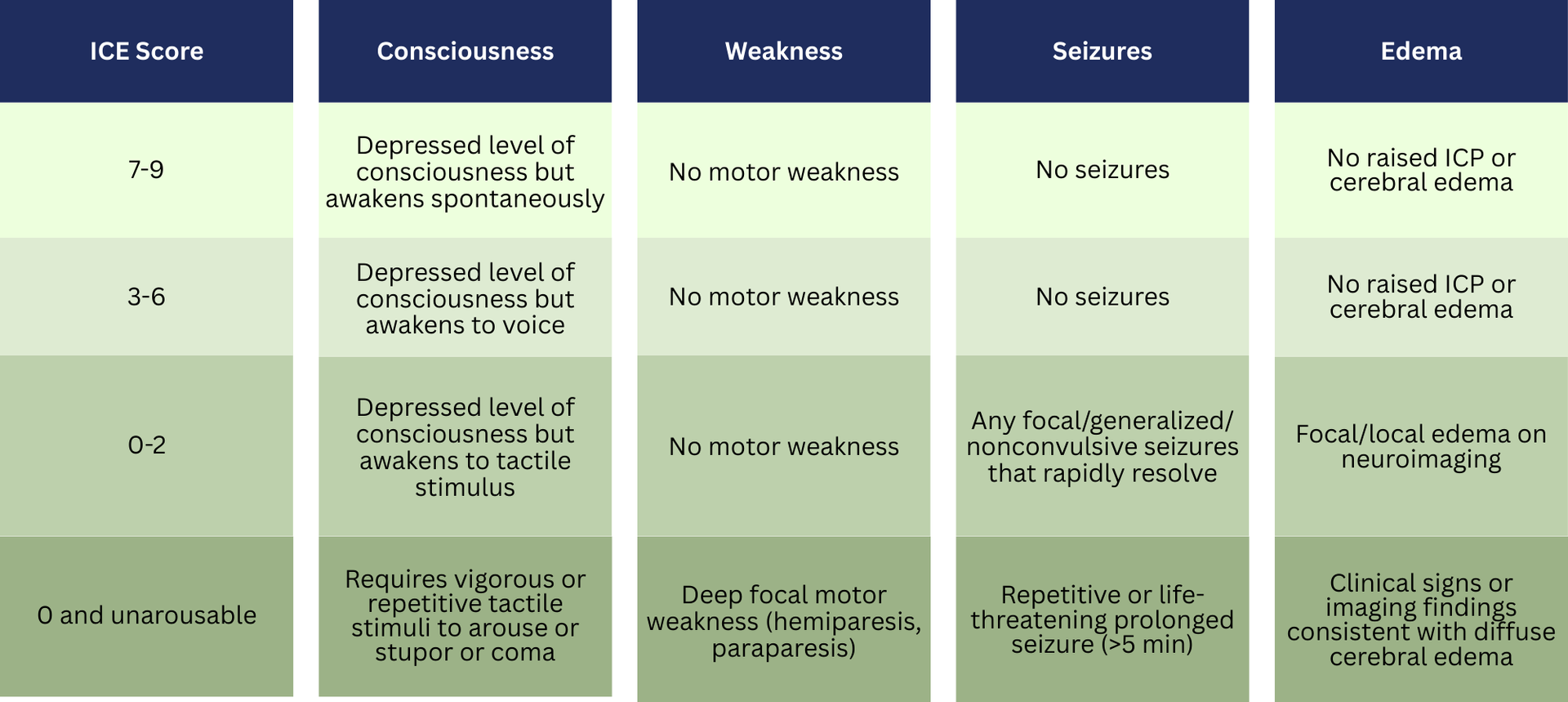
Documentation should clearly reflect the ICANS grading ICE score, which directly affects code assignment and severity classification.
Treatment Considerations Supporting Documentation
Common therapies for ICANS include:
- Supportive care
- Antiseizure agents or benzodiazepines
- Corticosteroids (e.g., dexamethasone)
- ICU care for severe neurotoxicity
- Tocilizumab only if CRS is present
Treatment details often help CDI professionals validate ICANS severity and grade.
ICD-10-CM Codes for ICANS
Immune effector cell–associated neurotoxicity syndrome, G92.0
- ICANS, grade 1: ICD-10 Code: G92.01
- ICANS, grade 2: ICD-10 Code: G92.02
- ICANS, grade 3: ICD-10 Code: G92.03
- CC/MCC: CC
- ICANS, grade 4: ICD-10 Code: G92.04
- CC/MCC: CC
- ICANS, grade 5: ICD-10 Code: G92.05
- CC/MCC: CC
- ICANS, unspecified: ICD-10 Code: G92.00'
Coding Note: Always report the underlying condition, immunotherapy used, and related complications (e.g., CRS) in addition to the ICANS diagnosis.
Explore More CDI Insights
For additional CDI Tips, regulatory updates, and documentation guidance: UASI's CDI Tips and Scenarios

Alyce Reavis, RN,MSN,CCDS,CCS
Senior CDI Educator, Consulting Services at UASI
Drawing on clinical experience in adult, pediatric, and neonatal acute care, Alyce brings valuable insight to CDI education and documentation improvement. She holds an MSN in Leadership/Education along with CCDS, CCS, and AHIMA’s outpatient CDI micro credential, supporting health systems in strengthening documentation accuracy, quality reporting, and reimbursement integrity. Passionate about truthful, clinically aligned health records, she helps organizations ensure documentation reflects true patient acuity. She is a past presenter for the ACDIS National Convention, Local chapter meetings, and the ACDIS Virtual Best Practices conference.
Works Cited
Centers for Medicare & Medicaid Services. Official Guidelines for Coding and Reporting. Available at https://www.cms.gov
Dietrich, J., & Frigault, M. J. Immune effector cell–associated neurotoxicity syndrome (ICANS) and other neurologic toxicities of CAR-T cell and related therapies. UpToDate, 2025. Available at https://www.uptodate.com/contents/immune-effector-cell-associated-neurotoxicity-syndrome-icans-and-other-neurologic-toxicities-of-car-t-cell-and-related-therapies
Prescott, L., & Manz, J. ACDIS CDI Pocket Guide – Inpatient. Available at https://acdis.org/