May 12, 2025
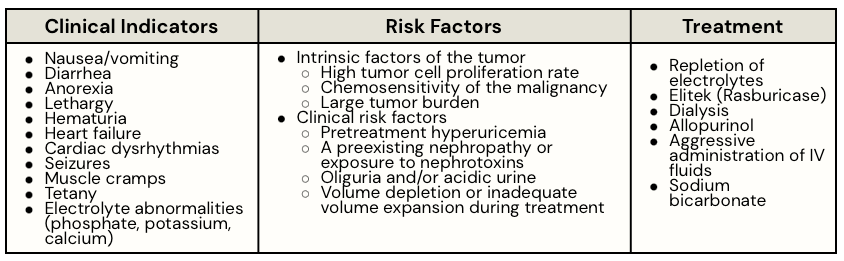
Definition: Tumor lysis syndrome (TLS) is an oncologic emergency caused by massive tumor cell lysis and the release of large amounts of potassium, phosphate, and uric acid into the systemic circulation. Deposition of uric acid and/or calcium phosphate crystals in the renal tubules can result in acute kidney injury.
Cairo-Bishop Definition and Grading System
- The Cairo-Bishop definition provides specific laboratory criteria for the diagnosis of TLS both at presentation and within seven days of treatment. There is also a grading system to define the degree of severity of TLS.
- Laboratory TLS: any two or more abnormal serum values for uric acid, potassium, calcium, or phosphorus
- Clinical TLS: grading system for severity of TLS in patients with laboratory TLS was based on the degree of elevation in serum creatinine, the presence and type of cardiac arrhythmia, and the presence and severity of seizures
ICD-10-CM Codes and Current Coding Advice
- E88.3, Tumor Lysis Syndrome
- Classifies as an MCC when reported as a secondary diagnosis
- When applicable, report an additional code to report adverse effect to identify the drug (T45.1X5)
- Prior advice suggests when TLS and CRS are present together that E88.3 and R65.10, SIRS of non-infectious origin, w/o acute organ dysfunction would be reported. However, there are now specific codes to report CRS (D89.831-D89.839)
CDI Clinical Scenario:
- HPI: A 25‐year‐old male with newly diagnosed high‐grade Burkitt lymphoma was admitted for induction chemotherapy. He had bulky disease with elevated lactate dehydrogenase (LDH) and an initial workup that documented dehydration. Baseline renal function was normal (serum creatinine 0.9 mg/dL)
- On admission, the patient’s workup noted high tumor burden and risk factors for tumor lysis syndrome (TLS), including elevated LDH (2.5× ULN) and a large mediastinal mass. Prophylactic measures were initiated with aggressive intravenous hydration and oral allopurinol.
- Chemotherapy Initiation & Onset of TLS: Approximately 18 hours after starting a multiagent chemotherapy regimen (cyclophosphamide, vincristine, and prednisone), the patient developed nausea, vomiting, and left flank pain. His urine output declined, and he reported a new-onset weakness.
- Laboratory Findings:
- Uric acid: 12 mg/dL (elevated)
- Potassium: 5.8 mmol/L
- Phosphorus: 8.0 mg/dL
- Calcium: 7.0 mg/dL (low)
- Creatinine: increased from 0.9 to 1.8 mg/d
- Provider documentation upon transfer to the ICU:
- “The patient was transferred to the intensive care unit for close monitoring and telemetry. Treatment was escalated to include aggressive IV hydration, a single dose of IV rasburicase (administered four hours before planned continuation of chemotherapy), and supportive electrolyte management. Documentation noted that allopurinol was held once rasburicase therapy was initiated”
CDI Query Example:
Dear Dr. _____,
Using your medical judgment, please clarify the condition that caused ICU admission for this patient.
- Tumor lysis syndrome
- Other etiology of clinical indicators (please specify)
Clinical Indicators:
- Newly diagnosed high‐grade Burkitt lymphoma admitted for induction chemotherapy with initial labs indicating dehydration.
- Approximately 18H after chemo, developed nausea, vomiting, and left flank pain. Urine output declined, and he reported new-onset weakness.
- Labs: Uric acid: 12 mg/dL (elevated); Potassium: 5.8 mmol/L; Phosphorus: 8.0 mg/dL; Calcium: 7.0 mg/dL (low); Creatinine: increased from 0.9 to 1.8 mg/d
- H&P states patient was transferred to the intensive care unit for close monitoring and telemetry. Treatment was escalated to include aggressive IV hydration, a single dose of IV rasburicase (administered four hours before planned continuation of chemotherapy), and supportive electrolyte management. Documentation noted that allopurinol was held once rasburicase therapy was initiated.
References:
- Centers for Medicare and Medicaid Services. ICD-10-CM Official Coding Guidelines for Coding and Reporting FY’ 2025. www.cms.gov.
- AHIMA, ACDIS (2022). Guidelines for Achieving a Compliant Query Practice (2022 Update).
- American Hospital Association. (2020). Coding Clinic ICD-10-CM/PCS, First Quarter 2020; Page 37
- Larson, R. (2024). Tumor Lysis Syndrome: Pathogenesis, clinical manifestations, definition, etiology and risk factors. UpToDate.
- Larson, R. (2022). Tumor Lysis Syndrome: Prevention and Treatment. UpToDate.