June 30, 2025
Coding Dual-Chamber Leadless Pacemakers in ICD-10-PCS
Key Coding Considerations for Dual-Chamber Leadless Pacemaker Devices
Leadless pacemakers are no longer limited to single-chamber technology. Dual-chamber leadless pacemakers are now available and increasingly utilized, offering expanded pacing capabilities while maintaining the benefits of a leadless system.
From a clinical perspective, dual-chamber leadless pacemakers are designed to improve patient experience by reducing device-related complications, minimizing pocket-related infections, and eliminating lead-associated risks. These devices are also designed to be retrievable, which has contributed to broader adoption in appropriate patient populations.
Coding Considerations
When coding dual-chamber leadless pacemaker implantation procedures, several key factors must be considered.
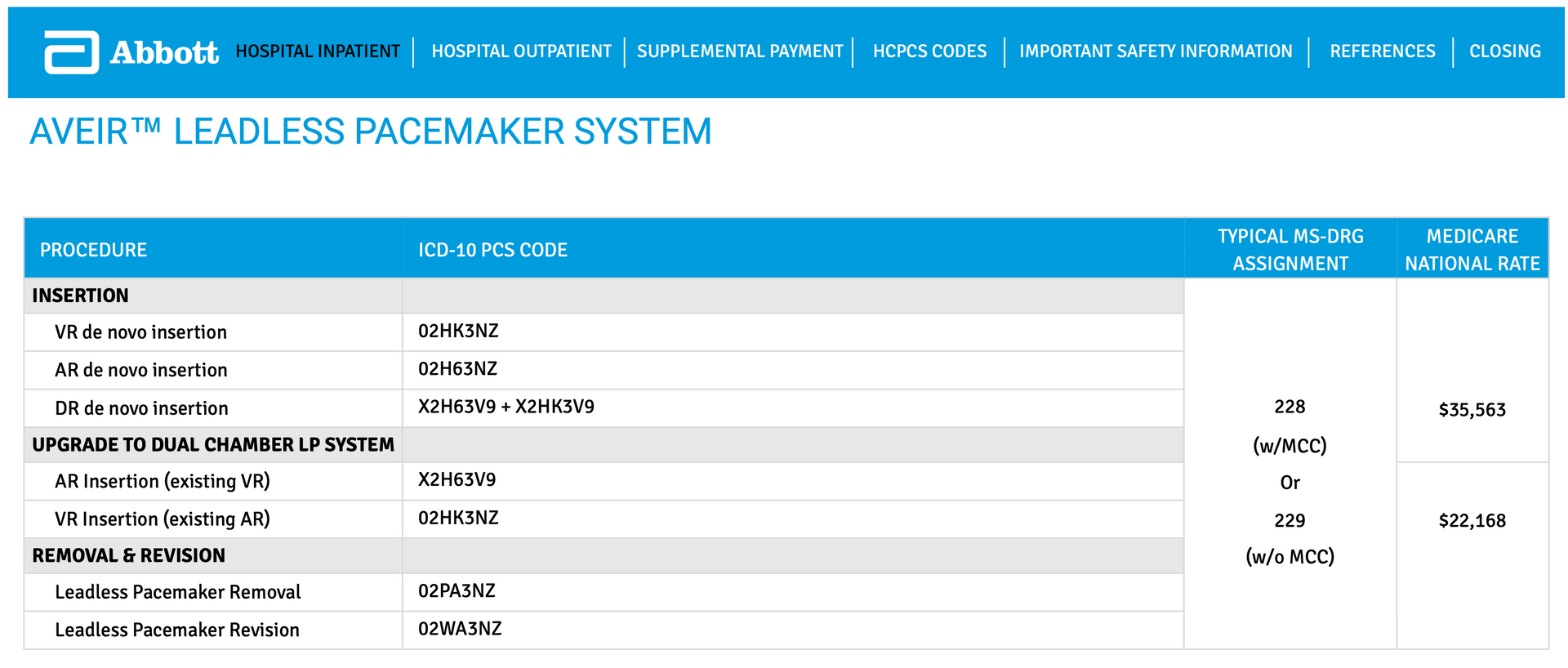
First, dual-chamber leadless pacemakers are classified as new technology and must be coded accordingly within ICD-10-PCS. Coders should verify that the procedure meets new technology criteria as outlined in current guidance.
Second, unlike traditional single-device systems, dual-chamber leadless pacemakers involve the implantation of two separate devices, typically one placed in the right atrium and one in the right ventricle. Each device represents a distinct implantation and must be reported separately.
Accurate code assignment requires careful review of the operative report to identify:
- The specific cardiac chamber for each device
- The approach used for implantation
- The device type and new technology designation
Coding Clinic guidance and manufacturer resources provide clarification on the appropriate ICD-10-PCS tables and code selection for dual-chamber leadless pacemaker systems. Coders should reference these materials to ensure complete and compliant reporting.
Kendra Adams, RHIT
Senior Consultant, Audit at UASI
With a background in health information technology and ICD-10 coding, Kendra Adams serves as a Senior Consultant in Audit at UASI. She contributes clear, practical coding tips rooted in real-world audit work to help coders improve accuracy, documentation quality, and compliant code assignment.
Works Cited
American Hospital Association. (2023). ICD-10-PCS coding for leadless pacemaker systems. Coding Clinic for ICD-10-PCS, Fourth Quarter 2023, pp. 60–61.
Abbott. (2023). AVEIR single and dual chamber leadless pacemaker systems. Abbott Laboratories. Available at: https://www.cardiovascular.abbott